
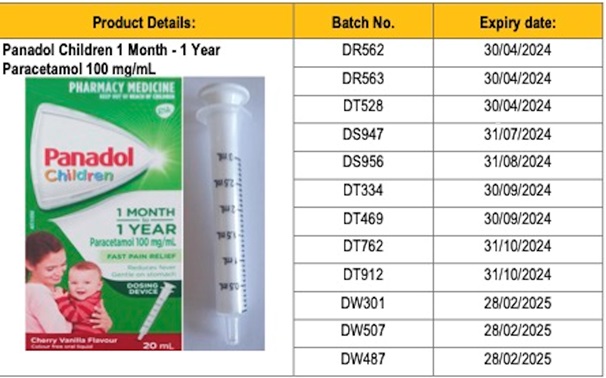
The Health Ministry has issued a recall for 14 batches of Panadol Children 1 Month – 1 Year Paracetamol 100mg/ml oral liquid bottle.
The ministry says the recall is based on a product defect correction alert from the United States Therapeutic Goods Administration (TGA), with the assistance of Haleon Australia Pty Limited.
The ministry says difficulties may be experienced when using the supplied dosing syringe.
“The dosing syringe in the batches in the scope of this recall may experience a performance problem (stiffness impacting the ability to control the delivery of the medicine) which may pose a risk of choking to babies,” the Health Ministry said.
The ministry says there is no concern around the liquid medicine itself. The component impacted is the syringe.
The problem with the syringe is not linked to all batches of the Panadol liquid. The only affected batches are:
Advice to Consumers:
Dispose of the supplied syringe, and seek an alternative syringe if product held is an affected batch.
Advice to Pharmacies:
Inspect all stock immediately and quarantine stock affected to prevent further use. Return stock to wholesaler for a credit to be issued by 10 May 2024. Ensure alternate 1mL syringes are made available to consumers at no cost. Pharmacies will be reimbursed by Haleon for supplying these syringes.
Advice to Wholesalers:
Inspect all stock immediately and quarantine affected stock to prevent further use. No further stock of these batch numbers is to be distributed to pharmacies. Pharmacies are being asked to remove stock from shelves and return to wholesaler for credit. Refunds for wholesalers will be arranged when the response form is returned. Replacement stock can be ordered via the usual ordering process. (It should be noted that Haleon does not export this product, hence customers that import this product are to contact their supplier.)
This information has been published in the TGA’s searchable database, the System for Australian Recall Actions (SARA): https://apps.tga.gov.au/Prod/sara/arn-detail.aspx?k=RC-2024-RN-00303-1 9.
Further clarifications can be made to the Medicines Regulatory Authority (MRA), email: [email protected] or phone: 8921660 with regards to this issue or any other that is similar in nature.